2020 – A year in axSpA research! Part one
Hi everyone – if you are new to the blog, then welcome! I hope that everyone is keeping well and having a good weekend.
Over the next couple of blog posts, we are going to run through some of the key research highlights for axial spondyloarthritis (axSpA) over the past year. And oh, what a year it has been! This first blog post will provide updates from January 2020 – March 2020.
We hope you enjoy reading through the study overviews, and as always, please feel free to get in touch with any questions by emailing Rosie at rosie.barnett1@nhs.net, and she will be happy to discuss with you. If you are on twitter, you can also follow **https://twitter.com/RosieBarnett95 ** for regular research and Project Nightingale updates, including notifications for new blog posts!
If you yourself are interested in writing a blog post for us, we would also love to hear from you! Not only regarding your potential experiences with Project Nightingale, but also regarding your general experiences with axSpA. Again, please email Rosie above, or get in touch using hello@projectnightingale.org.
Thank you for reading and have a good rest of the weekend everyone!
Best wishes,
The Project Nightingale Team
New biologic drug treatment for axSpA - an update on ixekizumab
COAST-V and COAST-W – efficacy and safety in individuals with radiographic axSpA (i.e. ankylosing spondylitis), Annals of the Rheumatic Diseases, Volume 79, Issue 2, February 2020, Pages 179-185, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7025731/
COAST-X – efficacy and safety in individuals with non-radiographic axSpA, Lancet, Volume 395, Issue 10217, January 2020, Pages 53-64, https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32971-X/fulltext
Background on biologics for axSpA
Currently in the UK, NICE (National Institute for Health and Care Excellence) recommend the tumour necrosis factor alpha (TNF-alpha – an inflammatory molecule produced by cells of the immune system) inhibitors, adalimumab, certolizumab pegol, etanercept and golimumab as treatment options for adults with severe active axSpA whose disease has responded inadequately to, or who cannot tolerate non-steroidal anti-inflammatory drugs (NSAIDs). Infliximab is also recommended for adults with severe active ankylosing spondylitis specifically. Biosimilar versions of adalimumab, etanercept and infliximab are available (i.e. biological products that are very similar to the reference biologic – no clinically meaningful differences in terms of safety, purity, and potency). NICE also recommends the interleukin-17A (IL-17A – like TNF-alpha, an inflammatory molecule produced by cells of the immune system) inhibitor secukinumab as an alternative to, or after inadequate response to TNF-alpha inhibitors for individuals with severe ankylosing spondylitis. Although secukinumab is yet to be licensed for use in non-radiographic axSpA in the UK, in June of this year, it was approved in the US. NICE will publish guidance on this in the UK on 19th May 2021 (previously delayed due to COVID).
Ixekizumab
Ixekizumab is another inhibitor of IL-17A, currently approved for use in moderate-to-severe plaque psoriasis and psoriatic arthritis with moderate-to-severe plaque psoriasis. It is not currently authorised for the treatment of axSpA in the UK.
Early this year, we saw publication of the 52-week results of three significant clinical trials for ixekizumab - COAST-V and COAST-W assessing efficacy and safety in individuals with radiographic axSpA (i.e. ankylosing spondylitis) and COAST-X assessing efficacy and safety in individuals with non-radiographic axSpA. The findings from these trials excitingly suggest that ixekizumab could be a longer-term treatment option for patients with axSpA, regardless of prior experience with TNF inhibitors. The additional improvement in adalimumab-treated patients after switching to ixekizumab is of particular interest and deserves further exploration.
Indeed, ixekizumab was just this year granted approval by the FDA in the US for treatment of both radiographic and non-radiographic axSpA! In the UK, unfortunately the NICE discussions/appraisal of ixekizumab has been delayed due to COVID. However, this is expected to be published on the 19th May 2021.
A quick word on JAK inhibitors…
The inhibition of Janus Kinase (JAK) molecules may also represent a future treatment strategy for axSpA. An article published in October of this year discusses this in detail (https://www.frontiersin.org/articles/10.3389/fimmu.2020.591176/full). Although the data from three clinical trials of JAK inhibitors in active ankylosing spondylitis are very promising, studies evaluating patients who have failed TNF inhibitors or anti-IL-17 therapy will be of great interest to place JAK inhibitors in the treatment algorithm of axSpA. Other interesting issues are head-to-head comparisons with TNF inhibitors and efficacy in non-radiographic axSpA. Long-term safety results are also currently awaited.
The authors of the October article suggested that new orally available JAK inhibitors will most likely soon be included in the treatment recommendations for axSpA and provide the clinician with options in patients who are not eligible or have contraindications to TNF inhibitors or anti-IL-17, such as allergic reactions, congestive heart failure or active IBD (anti-IL-17).
The impact of smoking on response to TNF inhibitors in axial spondyloarthritis: methodological considerations for longitudinal observational studies
Sizheng Zhao, Kazuki Yoshida, Gareth T Jones, David M Hughes, Sara K Tedeschi, Houchen Lyu, Robert J Moots, Daniel H Solomon, and Nicola J Goodson.
Arthritis care & research, 2020 Apr (Epub 2020 Mar 12), 72(4), 591–599. https://doi.org/10.1002/acr.23851
The present paper by Zhao and colleagues used longitudinal data from the British Society for Rheumatology Biologics Register for Ankylosing Spondylitis to compare the impact of smoking status on response to TNF-alpha inhibitor biologics medication. In this large UK cohort of axSpA participants, baseline smoking status was associated with significantly worse disease severity at baseline across all measures (disease activity, function, sleep, anxiety, depression). However, response to the first TNF-alpha inhibitor did not differ significantly according to baseline smoking status.
The authors conclude that prescribers should dispel any subconscious bias that smokers may not respond as well to treatment. Nevertheless, emphasise the importance of smoking cessation, particularly given the high burden of heart disease in rheumatic patients. Furthermore, smoking is associated with more severe disease (activity, functional impairment and radiographic progression); although it is not known whether cessation leads to improvement in disease outcome. Conflicting results from prior studies are likely explained by methodological differences.

Structural progression in axial spondyloarthritis
Krystel Aouad, Nelly Ziade, Xenofon Baraliakos.
Joint Bone Spine, 2020 Mar; 87(2):131-136. https://doi.org/10.1016/j.jbspin.2019.04.006
The following review article discussed structural progression in axSpA. See highlights in bullet points below:

High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): a multicentre randomised trial of 100 patients
Silje Halvorsen Sveaas, Annelie Bilberg, Inger Jorid Berg, Sella Arrestad Provan, Silvia Rollefstad, Anne Grete Semb, Kåre Birger Hagen, Melissa Woll Johansen, Elisabeth Pedersen, Hanne Dagfinrud
British Journal of Sports Medicine 2020 Mar; 54:292-297. https://bjsm.bmj.com/content/54/5/292.citation-tools
We of course all know that exercise is considered important in the management of axSpA. However, prior to the present study, the impact of high intensity exercises on disease activity was unknown.
In this randomised controlled trial of 100 participants with axSpA, aged from their 20s to 60s, high-intensity exercise over 3-months (in comparison to the no-intervention control group – instructed to maintain their usual physical activity level), reduced symptoms (pain, fatigue, stiffness), disease activity and also inflammation in people with axSpA. It also improved patients’ function and cardiovascular health.
This debunks concerns that high intensity exercise might exacerbate disease activity in people with axSpA. And suggests high intensity exercise as a potential management technique for individuals with axSpA.
Physical activity and sedentary behaviour and their associations with clinical measures in axial spondyloarthritis
Elaine H Coulter, Marie Therese McDonald, Sara Cameron, Stefan Siebert, Lorna Paul.
Rheumatology international 2020, 40(3), 375–381. https://doi.org/10.1007/s00296-019-04494-3
And another paper on physical activity and axSpA! This study by Elaine Coulter and colleagues investigated the relationship between sedentary behaviour and physical activity with clinical measures in 45 individuals with axSpA. Physical activity was measured using an activPAL3 (see image below) accelerometer, worn on the dominant thigh. The activePAL3 is waterproof and can be worn overnight.
Participants accumulated an average of 93.2 ± 41.5 min/day walking with an average of 7200 ± 3397 steps/day. The majority of the day (65%) was spent sitting, accumulated in prolonged bouts. Walking time and steps taken per day were associated with better physical function, exercise capacity and quality of life. Longer bouts of walking were associated with better spinal mobility, physical function and exercise capacity. Sedentary behaviour was associated with worse quality of life and exercise capacity.
These findings, as above, support the promotion of physical activity and reduction of sedentary behaviour in axSpA. Whereby, physical activity is associated with better function, exercise capacity and spinal mobility, while SB is associated with lower exercise capacity and poor quality of life.
Patients with axial spondyloarthritis report significant differences between men and women and high impact of the disease: large websurvey analysis.
Ibáñez Vodnizza SE , van Bentum RE , Valenzuela O , van der Horst-Bruinsma IE.
Joint Bone Spine 2020; 87: 315–9. https://doi.org/10.1016/j.jbspin.2020.02.004
In last weeks blog post, we discussed the special supplement addition of Rheumatology: including a piece by van der Horst-Bruinsma and colleagues on sex and gender differences in axSpA (see: https://www.projectnightingale.org/blogs/special-supplement-within-the-journal-rheumatology-1/). The following paper was included in this discussion. Whereby, females in Chile were found to have significantly higher disease activity, worse daily life impairment and worse work disability in comparison to males. However, despite this higher disease burden, were less likely to receive biologics (26% of males versus 16% of females).
Importantly, overall, the use of biologics was low, while the use of opiates such as codeine were worryingly high. As stated above, women used significantly less biologics despite reporting a worse disease state and work disability, which could be due to treatment inequity. This study therefore reveals the need to improve treatment access and gender inequality in Chilean axSpA.
Differences between females and males in axial spondyloarthritis: data from a real-life cross-sectional cohort
Hmy de Jong, J E Paramarta, Jjh de Winter, Dlp Baeten, Mgh van de Sande.
Scandinavian Journal of Rheumatology 2020, 49(1), 28-32. https://doi.org/10.1080⁄03009742.2019.1627410
As above, the present study looked at sex differences in axSpA, but in a large cohort (>300 individuals) in the Netherlands. In this real-life cohort of people with axSpA, although males more often had structural damage on X-rays, females had similar disease with regard to SpA features and at least equal disease activity parameters compared to males.
Demographic and clinical differences between ankylosing spondylitis and non-radiographic axial spondyloarthritis: results from a multicentre retrospective study in the Lazio region of Italy
Maria Sole Chimenti, Paola Conigliaro, Luca Navarini, Francesca Maria Martina, Giusy Peluso, Domenico Birra, Paola Sessa, Michele Anzidei, Palma Scolieri, Vincenzo Bruzzese, Gianluca Santoboni, Paolo Cardello, Elisa Gremese, Antonella Afeltra, Guido Valesini, Gian Domenico Sebastiani, Roberto Perricone, Rossana Scrivo.
Clinical and Experimental Rheumatology 2020, 38(1), 88-93. https://pubmed.ncbi.nlm.nih.gov/31140397/
It is important to recognise that axSpA is currently thought of as a spectrum of disease (see our previous blog post: https://www.projectnightingale.org/blogs/axial-spondyloarthritis-what-is-axspa-and-how-does-it-relate-to-ankylosing-spondylitis/ and Axial spondyloarthritis: time to stop the split Nature review article summary below). However, the present study aimed to identify signature features in ankylosing spondylitis versus non-radiographic axSpA.
210 people with axSpA were included in the present study: 65.2% with radiographic axSpA (ankylosing spondylitis – AS) and 34.7% with non-radiographic axSpA (nr-axSpA). When comparing the two groups, AS patients had longer disease duration, were older, were more frequently males, had a greater diagnostic delay and a higher body mass index (a measure of weight in comparison to height) than the nr-axSpA patients.
The peripheral joints of the nr-axSpA patients were more frequently involved, had higher frequency of inflammatory bowel disease, higher markers of inflammation (CRP) and lower frequency of HLA-B27 positivity. TNF inhibitors were used in 87.8% patients with AS and 78.3% with nr-axSpA. Significant differences were also found between the AS and nr-axSpA groups on MRI (imaging).
This work highlights distinctive features in individuals with AS and nr-axSpA. The authors furthermore suggest that imaging should be crucial in guiding the choice of treatment in order to control disease activity and inflammation.
The role of obesity on inflammation and damage in spondyloarthritis: a systematic literature review on body mass index and imaging
Maria Sole Sibel Bakirci, Janet Dabague, Lihi Eder, Dennis McGonagle, Sibel Zehra Aydin.
Clinical and Experimental Rheumatology Jan-Feb 2020, 38(1), 144-148. https://pubmed.ncbi.nlm.nih.gov/31074718/
In the present article, Bakirci and colleagues conducted a systematic review of all literature on axSpA up to February 2017, in order to evaluate the effect of obesity and/or body mass index (BMI) on radiographic findings of spondyloarthritis (SpA) for both axial and peripheral inflammation and damage.
A higher BMI was linked to both increased axial and peripheral new bone formation and entheseal (where tendons or ligaments attach to bone) inflammation by imaging, as supported by the limited number of studies in the literature. Unfortunately, its effect on the sacroiliac joint and spinal inflammation is not clear as MRI studies are lacking.
Other articles worth a mention…
This blog post is already incredibly long, so we won’t go into huge detail about the following studies. However, thought that these were definitely worth a mention! We have added links to the full-text publications, where available, in case you are interested in looking into this further.
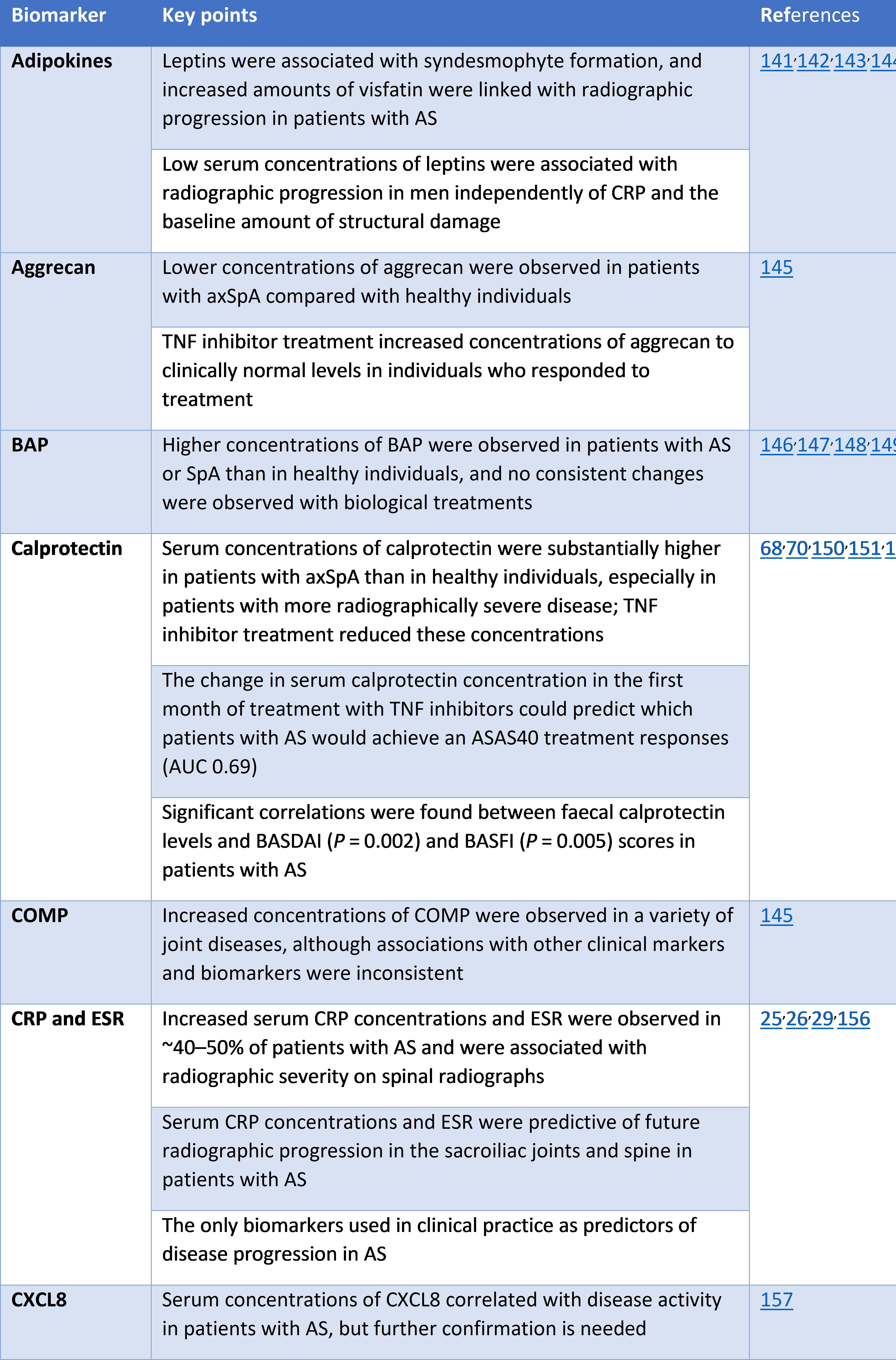
Biomarkers in axSpA
Interestingly, between January and March of this year, we saw two publications which identified potentially interesting new molecules that may be implicated in axSpA; with potential to be used as future biomarkers (biomarkers in axSpA are currently lacking – see our review article in last month’s supplement addition of Rheumatology for more information https://academic.oup.com/rheumatology/article/59/Supplement_4/iv25/5923444).
A study by Troldborg and colleagues looked at blood plasma levels of inflammatory molecules in a specific inflammatory pathway of the innate immune system. Although appearing not to be associated with disease activity (potentially due to the cross-sectional nature of the study – blood plasma levels not measured over time), H-ficolin and L-ficolin protein blood plasma concentrations were found to be elevated in axSpA patients regardless of time since diagnosis. The proteins H-ficolin and L-ficolin may therefore represent diagnostic biomarkers for patients with axSpA and should be further evaluated. See the article in full for more detail: https://onlinelibrary.wiley.com/doi/full/10.1111/cei.13374?saml_referrer.
Another study looked at the protein S100A4 – a molecule known to regulate bone formation. Interestingly, this is the first study showing elevated circulating levels of S100A4 in patients with axSpA. Particularly in early stages of the disease prior to spinal involvement. The authors therefore suggest a potential role for S100A4 in the development of axSpA! See the full article here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6993388/.
Gut involvement in axSpA
Two studies were published between January and March 2020 looking at gut involvement in axSpA.
Inflammatory bowel disease (IBD) is a well-known extraarticular feature of spondyloarthritis (SpA). In a cohort of individuals with early SpA, IBD was diagnosed in ~5% of individuals and was associated with poor outcome, familial history of IBD, absence of HLA-B27, and fulfillment of modified New York criteria (criteria for ankylosing spondylitis, specifically; https://www.jrheum.org/content/47/3/349.long).
Irritable bowel syndrome (IBS) is distinct from IBD and is thought to arise through mechanisms similar to fibromyalgia, common in axSpA (affecting up to 25% versus ~2% in the general population). However data for IBS in axSpA remain sparse. A study published by the BMJ group found that gut symptoms meeting IBS criteria were significantly more frequent among patients with axSpA (https://ard.bmj.com/content/79/1/159.long).
Articles already discussed in prior blog posts…
Several super interesting articles published in print in early 2020, were already published online towards the end of 2019. And have therefore been discussed in prior blog posts. As such, we have copied and pasted the relevant articles from older blog posts below, if you are interested…
Pre-prints October 2019 - https://www.projectnightingale.org/blogs/october-axspa-research-highlights/
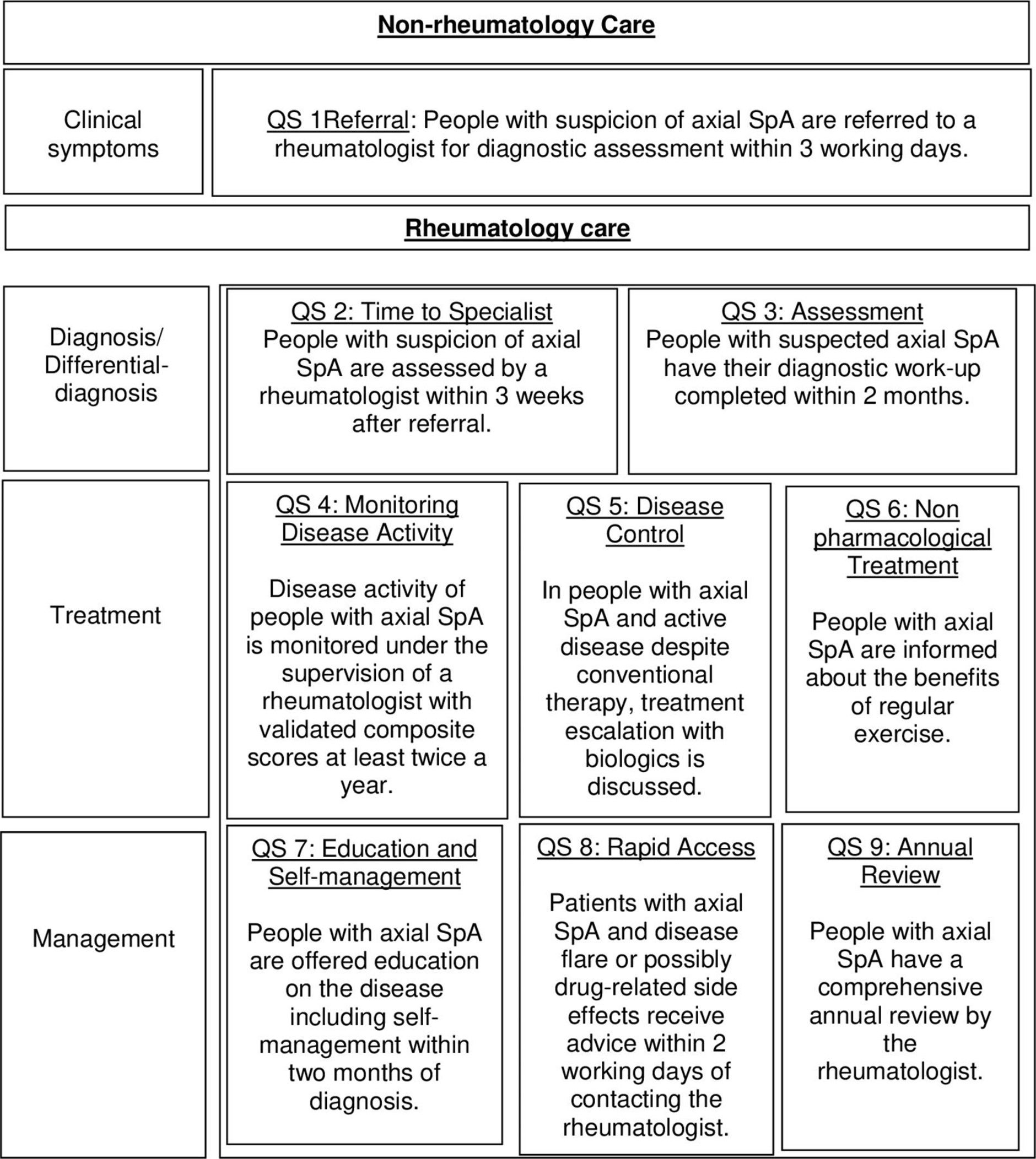
Development of ASAS quality standards to improve the quality of health and care services for patients with axial spondyloarthritis. Journal article published in BMJ Annals of the Rheumatic Diseases, 11th October. Uta Kiltz and colleagues. https://ard.bmj.com/content/early/2019/10/30/annrheumdis-2019-216034.long#ref-2
This month, excitingly the Assessment of SpondyloArthritis International Society (ASAS) have published a set of quality standards (QS) to help improve the quality of healthcare provided worldwide to those affected by axSpA. The ASAS task force, consisting of 20 rheumatologists, 2 physiotherapists and 2 people with axSpA, developed this set of QS using a stepwise approach; proposing 34 potential key areas for quality improvement, which were then commented on by 140 ASAS members and people with axSpA, leading to identification of 3 additional areas for consideration. Eventually, after much discussion, 5 key areas were determined as most important to determine quality of care: referral including rapid access, rheumatology assessment, treatment, education/self-management and comorbidities. Finally, nine QS were agreed on and endorsed by the whole ASAS membership. These 9 QS are outlined below. The aim is now to implement each of these quality standards at a national level, for local quality improvement.
Axial spondyloarthritis: time to stop the split 10 years on. Review article published in Nature Reviews Rheumatology, 7th October. Xabier Michelena & Helena Marzo-Ortega. https://www.nature.com/articles/s41584-019-0331-6
This article was in response to the recent publication of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network (ACR-SAA-SPARTAN) recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis, in Arthritis Rheumatology – https://doi.org/10.1002/art.41042.
The present article, very much focuses on the fact that the recommendations refer to non-radiographic (nr-axSpA) and radiographic axSpA (ankylosing spondylitis – AS) individually, rather than as a unified ‘spectrum’ of disease (as discussed in our previous blog post here: https://www.projectnightingale.org/blogs/axial-spondyloarthritis-what-is-axspa-and-how-does-it-relate-to-ankylosing-spondylitis/), in addition to discussing the recommendations in more detail. Important to note was the discussion regarding the ACR–SAA–SPARTAN recommendations rightly warning about the current limitations of MRI in disease monitoring (as touched upon above), particularly in view of the limited data available on the correlation between MRI lesions and treatment response or on the significance of subclinical inflammatory changes.
The authors conclude that: the 2019 ACR–SAA–SPARTAN recommendations provide an update for the rheumatology community with useful advice on how to manage axSpA and the use of new pharmacological therapies for this condition. Despite being formulated as if treating two distinct disease subsets (AS and nr-axSpA), similarities in the available evidence, including the treatment guidance devised, suggest that AS and nr-axSpA are indeed part of the same clinical entity and that management strategies should therefore be unified and aimed at the whole spectrum of axSpA. The integration of MRI in the evaluation of axSpA might be of value in some instances; however, research efforts are still needed to identify potential biomarkers and immune-phenotypes that could guide informed treatment stratification and our overall understanding of axSpA.
Determining factors related to poor quality of life in patients with axial spondyloarthritis: results from the British Society for Rheumatology Biologics Register (BSRBR-AS). Journal article published in BMJ Annals of the Rheumatic Diseases, 29th October. Gary Macfarlane and colleagues. https://ard.bmj.com/content/early/2019/10/29/annrheumdis-2019-216143.long
The following study involved analysing data from the British Society for Rheumatology Biologics Register in Ankylosing Spondylitis (BSRBR-AS) – thank you to those of you who contributed! The results confirmed that poor quality of life, high disease activity and poor function in people with axSpA, is independently influenced by sleep disturbance, mood and widespread pain – again highlighting these factors as key areas that should be targeted in the management of axSpA. Currently, sleep disturbance, mood and widespread pain are not acknowledged in European guidelines for managing the condition as targets to improve quality of life. However, there is an increasing amount of research highlighting the importance of these factors, including Project Nightingale, which will hopefully in the near future lead to an increasing focus on these aspects of the condition throughout the treatment process.
Pre-print November 2019 - https://www.projectnightingale.org/blogs/november-axspa-research-highlights/
Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. BMJ Annals of the Rheumatic Diseases, February 2020, Issue 79. Epub November 19th. Xenofon Baraliakos and colleagues. https://ard.bmj.com/content/early/2019/11/27/annrheumdis-2019-215553.long
As highlighted in our October axSpA Research Highlights blog post (https://www.projectnightingale.org/blogs/october-axspa-research-highlights/), MRI assessment has been transformative in the diagnosis of axSpA and has allowed for much earlier detection of structural changes and inflammation. However, interpretation of MRI is challenging and some changes lack specificity for axSpA. For example, bone marrow oedema has previously been shown to be present in 20-40% of people with nonspecific back pain or in healthy individuals; 35% of 20 recreational runners before running and 30% after running; 41% of 22 elite ice hockey players; in addition to 57.1% of women with postpartum back pain less than 3 months after pregnancy.
This particular study from Xenofon Baraliakos and colleagues highlights this issue once again, whereby the authors found a high frequency of MRI changes suggestive of axSpA in a large population cohort of 793 volunteers. Specifically, sacroiliac joint bone marrow oedema was identified in 136 (17.2%) individuals, vertebral corner bone marrow oedema found in 218 (27.5%), and fatty lesions present in 645 (81.4%). Furthermore, such changes appeared to occur more frequently in older age groups, perhaps suggesting mechanical factors could play a role. This therefore supports existing evidence suggesting that caution is needed when using MRI findings to support a diagnosis of axSpA, especially in the absence of other clear clinical symptoms. Indeed, we may soon see an update in the ASAS criteria for diagnosing axSpA, in terms of the imaging arm and definitions of MRI findings suggestive of axSpA.
Clinical manifestations, disease activity and disease burden of radiographic versus non-radiographic axial spondyloarthritis over 5 years of follow-up in the DESIR cohort. BMJ Annals of the Rheumatic Diseases, February 2020, Issue 79. Epub November 19th. Clementina López-Medina and colleagues. https://ard.bmj.com/content/early/2019/11/29/annrheumdis-2019-216218
In the present study, 669 people with axSpA from the DESIR cohort (a large, multicentre, longitudinal follow-up study of early inflammatory back pain in France) were included; of whom 185 (27.7%) were classified as having radiographic axSpA (r-axSpA, Ankylosing Spondylitis) and 484 were classified as non-radiographic axSpA (nr-axSpA). At baseline, as often anticipated, there was a higher prevalence of males in the r-axSpA cohort. The r-axSpA cohort also contained more smokers, with higher CRP levels than the nr-axSpA cohort. These characteristics are classically described as risk factors for structural damage in axSpA. HLA-B27 positivity and alcohol intake were also more frequent among people with nr-axSpA (as previously described in the DESIR cohort), with a higher prevalence of heel enthesitis and peripheral enthesitis (inflammation of the entheses, the sites where tendons or ligaments insert into the bone) at baseline. However, it is important to note that these peripheral manifestations might be artificially overrepresented among people with nr-axSpA because they help to diagnose nr-axSpA in the absence of radiographic evidence.
In the present study, the authors aimed to evaluate the incidence of first episodes of peripheral and extra-rheumatic manifestations between the two groups after diagnosis. For this reason, in the longitudinal analysis they decided to remove patients with a positive event (peripheral or extra-articular manifestations) at baseline to avoid the potential bias caused by the prevalence at the inclusion visit. Although the nr-axSpA group showed a higher prevalence of peripheral enthesitis at baseline, the incidence was similar between groups after the exclusion of patients with a positive event at the inclusion visit, supporting the suggestion that peripheral manifestations might be artificially overrepresented among people with nr-axSpA because they help to diagnose nr-axSpA in the absence of radiographic evidence.
Over time, the nr-axSpA group showed a greater disease burden, with higher scores in BASDAI and BASFI, poorer quality of life and a larger number of days of sick leave at the follow-up. The authors propose two theories for this: 1) a percentage of nr-axSpA patients may have concomitant fibromyalgia, as observed in a previous study by Moltó and colleagues; 2) other factors and patient characteristics may influence disease activity and disease burden. Therefore, the authors adjusted their analyses to take into account factors and patient characteristics that might lead to the higher scores observed among nr-axSpA group (ie, age, sex and biologics use). After adjusting for these variables, differences in disease burden between r-axSpA and nr-axSpA disappeared, whereby incidence of peripheral and extra-rheumatic manifestations (for example uveitis, psoriasis, dactylitis and other extra-spinal features associated with axSpA) remained similar between groups, in addition to patient-reported outcomes (for example BASDAI and BASFI) and days of sick leave.
These results suggest that both r-axSpA and nr-axSpA behave similarly over time, thereby supporting the concept of axSpA as one disease spectrum (see previous blog post: https://www.projectnightingale.org/blogs/axial-spondyloarthritis-what-is-axspa-and-how-does-it-relate-to-ankylosing-spondylitis/ and article in October research highlights post https://www.projectnightingale.org/blogs/october-axspa-research-highlights/). Interestingly, in an exploratory approach, the authors also found that several factors appeared to explain BASDAI over time; age, sex, education, radiographic sacroiliitis, use of anti-TNF medication and me