November AxSpA Research Highlights
Hi everyone, I hope you are all having a good week! Below is a summary of some of November’s axial spondyloarthritis (axSpA) research highlights, following on nicely from some of the themes identified in last month’s research highlight blog post; including evidence for axSpA as a single disease spectrum and caution when using MRI imaging to identify structural changes in axSpA, in addition to mention of the gut microbiome.
Have you read any interesting articles last month that we have missed, and you think should be included here? If so, please get in touch at rosie.barnett1@nhs.net and we can add them to our page.
Non‐Steroidal Anti‐Inflammatory Drugs As Potential Disease Modifying Medications In Axial Spondyloarthritis. Arthritis and Rheumatology, November 9th. Runsheng Wang and colleagues. https://onlinelibrary.wiley.com/doi/abs/10.1002/art.41164
While in recent years treatment options for axSpA have expanded due to the introduction of biologics medication, non-steroidal anti-inflammatory drugs (NSAIDs) remain the first line pharmacotherapy for people with axSpA. Many clinical trials support their use short-term and demonstrate efficacy for short-term reductions in disease activity. More contentious however is the evidence for their effectiveness in slowing disease progression – with two recent randomised controlled trials (randomised controlled trials often described as the scientific gold standard for studying interventions) publishing opposing findings. A comprehensive review published last month in Arthritis and Rheumatology discusses the clinical evidence for the potential effect of NSAIDs and biologics on radiographic progression in axSpA, in addition to exploring potential influence of NSAID use on bone formation.
With regards to NSAIDs specifically and the two randomised controlled trials mentioned above, one trial was with the selective COX-2 inhibitor celecoxib (COX-2 an enzyme involved in inflammatory pathways); whereby patients randomised to continuous use of celecoxib showed less radiographic progression after 2 years in comparison to patients randomised to on-demand use. Furthermore, progression with continuous treatment was greater in patients with elevated inflammatory markers (erythrocyte sedimentation rate or CRP). Meanwhile, the second trial investigated use of the non-selective NSAID diclofenac, utilising a similar study design to the celecoxib trial. However, continuous diclofenac use was associated with more progression over 2 years. There are however important caveats that should be considered when interpreting these results. For one, there was an absence of dose-effect (for example for the celecoxib trial, the average dose in the continuous group was only modestly higher than in the on-demand group, despite the significant difference in outcome). In addition to the lack of placebo-control group (it would be unethical to conduct placebo-controlled trials for 2 years), and potential imbalance of risk factors during randomisation possibly biasing treatment effects (for example, the continuous use group in the diclofenac trial had a higher proportion of smokers at baseline, although the impact of this is unclear).
To summarise, whether or not NSAIDs or biologics have a disease modification effect, or (more fundamentally) whether suppressing inflammation is sufficient to prevent bone formation in axSpA remains unclear. However, several studies investigating this are ongoing, and suggestions have been given by the authors of the present study with regards to the improvement of future studies exploring the progression of axSpA, including:
- Radiographic outcome measures: new imaging modalities – in recent years, 3D imaging modalities including full-dose computed tomography (CT) and low dose CT have been evaluated to improve the measurement of radiographic progression. Both methods have been shown to be more sensitive to changes than traditional scoring of progression using a measure known as mSASSS. To date, no randomised controlled trials have been reported using these new measures for disease modification effects.
- Participants: identifying subsets of disease – A handful of risk factors for radiographic progression have been identified from previous longitudinal studies, including male sex, HLA-B27 positivity, smoking status, elevated CRP, and presence of syndesmophytes (bony growths within ligaments) at baseline. These risk factors could therefore identify those people at highest risk of progression, whereby for these people, there would be a greater chance of detecting a change in radiographic progression over time. Treating people at high-risk of progression with minimal or full doses of NSAIDS and assessing progression with spinal CT may therefore be most likely to detect an NSAID effect.
- Identifying novel risk factors and potential interactions – recent cross-sectional studies have highlighted potential new risk factors for disease progression, including vitamin D and adipokines (cell signalling molecules secreted by adipose [fat] tissue), but these have not yet been included in longitudinal studies and their functions and potential interactions need to be better understood. Once defined, these could be included in future studies as covariates also and may aid our understanding of axSpA progression.
Beneficial long-term effect on leisure time physical activity level in individuals with axial spondyloarthritis: secondary analysis of a randomized controlled trial. Journal of Rheumatology, November 15th. Silje Halvorsen Sveaas and colleagues. http://www.jrheum.org/content/early/2019/11/08/jrheum.190317.long
As you will be aware, physical activity is suggested to have beneficial effects not only for our general health, but also specifically for axSpA. Recent secondary analysis from a randomised controlled trial explored the long-term effect of a 3-month exercise programme on leisure time physical activity in individuals with axSpA. The exercise group participated in a 3-month exercise programme while the control group received no intervention. Physical activity during leisure time was measured with a questionnaire (physically active = ≥1 hour/week with moderate/vigorous intensity physical activity), while disease activity was measured with the Ankylosing Spondylitis Disease Activity Scale (ASDAS, higher score=worst).
At 12-month follow-up, significantly more individuals in the exercise intervention group than the control group were physically active (29 [67%] vs. 13 [30%]) and exercised 2-3 times per week (25 [58%] vs. 15 [34]) and fewer exercised at light intensity (3 [8%] vs. 14 [44%]). The factors most associated with being physically active were participation in the exercise group and being physically active at baseline. However, still, few individuals continued on the intensive programme and there was no difference between the groups in disease activity after 12 months. This study highlights the difficulties regarding adherence to physical activity interventions, which definitely requires further study. As mentioned previously, at the Bath Spondyloarthritis Research Consortium (a collaboration of academic and clinical experts across the University of Bath and Royal National Hospital for Rheumatic Diseases), we are trying to better understand how we can effectively support people with axSpA when partaking in physical activity and further understand and encourage motivation for exercise. As always, we will keep you posted with any research updates here also!

Frequency of MRI changes suggestive of axial spondyloarthritis in the axial skeleton in a large population-based cohort of individuals aged <45 years. BMJ Annals of the Rheumatic Diseases, November 19th. Xenofon Baraliakos and colleagues. https://ard.bmj.com/content/early/2019/11/27/annrheumdis-2019-215553.long
As highlighted in our October axSpA Research Highlights blog post (https://projectnightingaleorg.wpcomstaging.com/2019/11/03/october-axspa-research-highlights/), MRI assessment has been transformative in the diagnosis of axSpA and has allowed for much earlier detection of structural changes and inflammation. However, interpretation of MRI is challenging and some changes lack specificity for axSpA. For example, bone marrow oedema has previously been shown to be present in 20-40% of people with nonspecific back pain or in healthy individuals; 35% of 20 recreational runners before running and 30% after running; 41% of 22 elite ice hockey players; in addition to 57.1% of women with postpartum back pain less than 3 months after pregnancy.
This particular study from Xenofon Baraliakos and colleagues highlights this issue once again, whereby the authors found a high frequency of MRI changes suggestive of axSpA in a large population cohort of 793 volunteers. Specifically, sacroiliac joint bone marrow oedema was identified in 136 (17.2%) individuals, vertebral corner bone marrow oedema found in 218 (27.5%), and fatty lesions present in 645 (81.4%). Furthermore, such changes appeared to occur more frequently in older age groups, perhaps suggesting mechanical factors could play a role. This therefore supports existing evidence suggesting that caution is needed when using MRI findings to support a diagnosis of axSpA, especially in the absence of other clear clinical symptoms. Indeed, we may soon see an update in the ASAS criteria for diagnosing axSpA, in terms of the imaging arm and definitions of MRI findings suggestive of axSpA.
Clinical manifestations, disease activity and disease burden of radiographic versus non-radiographic axial spondyloarthritis over 5 years of follow-up in the DESIR cohort. BMJ Annals of the Rheumatic Diseases, November 29th. Clementina López-Medina and colleagues. https://ard.bmj.com/content/early/2019/11/29/annrheumdis-2019-216218
In the present study, 669 people with axSpA from the DESIR cohort (a large, multicentre, longitudinal follow-up study of early inflammatory back pain in France) were included; of whom 185 (27.7%) were classified as having radiographic axSpA (r-axSpA, Ankylosing Spondylitis) and 484 were classified as non-radiographic axSpA (nr-axSpA). At baseline, as often anticipated, there was a higher prevalence of males in the r-axSpA cohort. The r-axSpA cohort also contained more smokers, with higher CRP levels than the nr-axSpA cohort. These characteristics are classically described as risk factors for structural damage in axSpA. HLA-B27 positivity and alcohol intake were also more frequent among people with nr-axSpA (as previously described in the DESIR cohort), with a higher prevalence of heel enthesitis and peripheral enthesitis (inflammation of the entheses, the sites where tendons or ligaments insert into the bone) at baseline. However, it is important to note that these peripheral manifestations might be artificially overrepresented among people with nr-axSpA because they help to diagnose nr-axSpA in the absence of radiographic evidence.
In the present study, the authors aimed to evaluate the incidence of first episodes of peripheral and extra-rheumatic manifestations between the two groups after diagnosis. For this reason, in the longitudinal analysis they decided to remove patients with a positive event (peripheral or extra-articular manifestations) at baseline to avoid the potential bias caused by the prevalence at the inclusion visit. Although the nr-axSpA group showed a higher prevalence of peripheral enthesitis at baseline, the incidence was similar between groups after the exclusion of patients with a positive event at the inclusion visit, supporting the suggestion that peripheral manifestations might be artificially overrepresented among people with nr-axSpA because they help to diagnose nr-axSpA in the absence of radiographic evidence.
Over time, the nr-axSpA group showed a greater disease burden, with higher scores in BASDAI and BASFI, poorer quality of life and a larger number of days of sick leave at the follow-up. The authors propose two theories for this: 1) a percentage of nr-axSpA patients may have concomitant fibromyalgia, as observed in a previous study by Moltó and colleagues; 2) other factors and patient characteristics may influence disease activity and disease burden. Therefore, the authors adjusted their analyses to take into account factors and patient characteristics that might lead to the higher scores observed among nr-axSpA group (ie, age, sex and biologics use). After adjusting for these variables, differences in disease burden between r-axSpA and nr-axSpA disappeared, whereby incidence of peripheral and extra-rheumatic manifestations (for example uveitis, psoriasis, dactylitis and other extra-spinal features associated with axSpA) remained similar between groups, in addition to patient-reported outcomes (for example BASDAI and BASFI) and days of sick leave.
These results suggest that both r-axSpA and nr-axSpA behave similarly over time, thereby supporting the concept of axSpA as one disease spectrum (see previous blog post: https://projectnightingaleorg.wpcomstaging.com/2019/10/17/axial-spondyloarthritis-what-is-axspa-and-how-does-it-relate-to-ankylosing-spondylitis/ and article in October research highlights post https://projectnightingaleorg.wpcomstaging.com/2019/11/03/october-axspa-research-highlights/ ). Interestingly, in an exploratory approach, the authors also found that several factors appeared to explain BASDAI over time; age, sex, education, radiographic sacroiliitis, use of anti-TNF medication and mean CRP.
Characteristics and burden of disease in patients with radiographic and non-radiographic axial Spondyloarthritis: a comparison by systematic literature review and meta-analysis. Rheumatic and Musculoskeletal Diseases, November 21st. Clementina López-Medina and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6890393/
Clementina López-Medina and colleagues, in addition to the study above, also completed a systematic review and meta-analysis of literature from 2009-2018 regarding the outcomes of people with radiographic and non-radiographic axSpA. 60 studies were included in the analyses. Due to the detail described above, I will not go into great detail here regarding the results, however essentially, based on this review, again people with r-axSpA and nr-axSpA appear to share a similar clinical presentation except for peripheral involvement, which is more prevalent among nr-axSpA. Except for a more impaired mobility in r-axSpA, both groups showed a comparable burden of disease, treatment modalities and treatment effect; again supporting the idea of axSpA as a single disease spectrum.
Associations between certolizumab pegol (Cimzia) serum levels, anti-drug antibodies and treatment response in patients with inflammatory joint diseases: data from the NOR-DMARD study. Arthritis Research Therapy, November 29th. Johanna Elin Gehin and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6883678/
TNF inhibitors such as certolizumab pegol (Cimzia) have substantially improved the management of inflammatory joint conditions such as axSpA. However, there remains a significant proportion of people who do not respond adequately to treatment. Lack of response has previously been associated with low drug levels and development of anti-drug antibodies and it has been suggested that therapeutic drug monitoring may help clinicians tailor treatment with biologics drugs. This may potentially reduce under and over-treatment and improve effectiveness, safety and cost-effectiveness of their administration.
In order to implement therapeutic drug monitoring, therapeutic target intervals need to be defined for predicting treatment response. Therapeutic target intervals have previously been reported for the biologics drugs adalimumab and infliximab, however these have usually been reported for people with rheumatoid arthritis only. In the present study, the authors determined a therapeutic target interval for certolizumab pegol; whereby levels of 20-40mg/L of certolizumab pegol in the blood were found to be associated with treatment response in inflammatory joint conditions – specifically, axSpA, psoriatic arthritis and rheumatoid arthritis. This is the first study to show this association for certolizumab pegol in axSpA and psoriatic arthritis.
Considerable variation was found between individuals for blood serum levels of the drug, despite having received the same standard dose – suggesting both potential over- and under-treatment. Of the three inflammatory conditions observed in this study, the association between drug blood serum levels and clinical response was most consistent for people with axSpA. Proportions of responders was lower in the over 40 mg/L group than in the 20–39.9 mg/L group for all three diagnoses. At first this may seem counterintuitive, but it has been suggested that non-responders with high drug levels in the blood may have lower amounts of the inflammatory molecule TNF in the blood, and therefore less binding of the drug occurs, resulting in large amounts of unbound drug. For people with large amounts of unbound drug in the blood, it may therefore be likely that their drug dose could be reduced without increasing the risk of disease worsening; which could importantly potentially reduce side effects, in addition to reducing costs, or may suggest that these people could benefit from switching to a biologic drug with a different mode of action. Indeed, for adalimumab, it has previously been shown that non-responders with high levels of unbound drug in the blood and no detectable anti-drug antibodies had higher probability of achieving response by switching to a drug with a different mode of action.
To summarise, this study found that blood serum levels of over 20mg/L of certolizumab pegol were associated with treatment response, however a serum level of over 40mg/L appeared to have no additional benefit. Anti-drug antibodies against certolizumab were associated with low drug levels and reduced treatment response. These results suggest that a therapeutic target interval of 20-40mg/L may be beneficial in inflammatory conditions, including axSpA. However the clinical significance of tailoring treatment by therapeutic drug monitoring should be further explored in randomised control trials.
Variations in gut microbial profiles in ankylosing spondylitis: disease phenotype-related dysbiosis. Annals of Translational Medicine, October. Zena Chen and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6861740/
Although this was technically published in the October issue of Annals of Translational Medicine, I thought it would be interesting to mention the present article – given the current surge in awareness and interest regarding gut microbiome research. Whereby, the microbiome is the genetic material of all the microbes – bacteria, fungi, protozoa and viruses – that live on and inside the human body.
It has previously been described that as many as 40-60% of people with axSpA may present with subclinical intestinal inflammation, whereby 5-10% of these people will progress into clinically established IBD throughout their condition. Increasing evidence appears to be emerging suggesting a link between gut dysbiosis and axSpA; including studies sequencing the microbiome of individuals with and without axSpA. The present study adds to this evidence, by investigating features of the gut microbiome in people with axSpA – while also exploring whether differences could be determined between the gut microbiome of individuals with peripheral and axial symptoms also.
In contrast to previous studies (Costello and colleagues reported a higher diversity while Breban and colleagues reported a decreased diversity in SpA patients), the authors found no significant difference in gut bacteria diversity between patients and healthy controls. Despite this, 11 genera of bacteria were found to be associated with axSpA, including the enrichment of Prevotella and Dialister and the depletion of Bacteroides, which were also observed in previous studies. The authors also observed a decrease abundance of _Ruminococcusgnavus in people with axSpA, especially in people with axial symptoms. However, Breban et al. reported that SpA patients possess a decrease abundance in _Ruminococcusgnavus. _Ruminococcusgnavus was reported with an association with IBD in previous study. The different findings between the present study and Breban and colleagues remains an open question, with a difference in gut inflammation status between the two study cohorts. This confusion reflects that more study and understanding is needed in this rapidly expanding field. Comamonas, a possible pathogen that may cause intestinal infection such as appendicitis showed a higher abundance people with axSpA. The authors then defined a statistical model that could accurately classify people with Ankylosing Spondylitis from healthy people, based on 8 genera of gut bacteria.
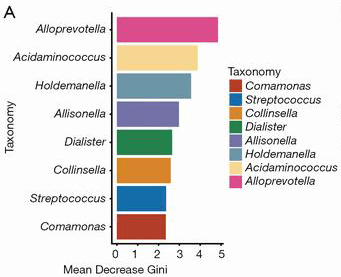
8 genera of bacteria used in the predictive model for identifying people with axSpA from healthy controls, ranked in descending order of importance to the accuracy of the model based on mean decrease Gini index
In terms of differentiating between people with axSpA who had axial and peripheral symptoms, a statistical model to differentiate between gut microbiomes showed low accuracy to classify between groups. Despite this, they found that _Prevotella2 was enriched in people with axial symptoms while Comamonas, Streptococcus and Collinsella were more enriched in people with peripheral symptoms.
To summarise, the study revealed specific alterations in the gut microbiomes in patients with different phenotypes of axSpA, and a classification model based on gut microbial features might provide a new direction for future clinical diagnosis and research into new treatments. However, some confusion still exists in the field and further research is needed to elucidate the role of the microbiome in axSpA.
If you made it to the end of the post then thank you for reading! Have a great week