2020 – A year in axSpA research! Part two
Introduction
Hi everyone,
I hope that you are all safe and well and enjoying this lovely Sunday morning!
Today’s blog post is part two in our 2020 research summary – providing updates from April 2020 – June 2020. While looking through the literature, it was impossible to squeeze everything from these 3 months into one blog post (too many interesting articles!). So, we have instead separated April - June into two posts. Today, we will be taking a look at 11 publications, published under 4 themes: physical activity, smoking, obesity and biomarkers.
As always, we hope you enjoy reading through the study overviews/ discussions. If you have any questions, or would like to talk through any of these studies in more detail, please feel free to get in touch by emailing our dedicated Project Nightingale researcher Rosie at rosie.barnett1@nhs.net and she will be happy to discuss with you (see September’s blog post for an introduction: https://www.projectnightingale.org/blogs/and-we-are-finally-back-with-the-regular-blog-posts/). If you are on twitter, you can also follow **https://twitter.com/RosieBarnett95 ** for regular research and Project Nightingale updates, including notifications for new blog posts!
If you yourself are interested in writing a blog post for us, we would also love to hear from you. Not only regarding your potential experiences with Project Nightingale, but also regarding your general experiences with axSpA. These can be posted anonymously, or with your name – whichever you prefer. Again, please email Rosie above, or get in touch using hello@projectnightingale.org. Here is a fantastic blog post published by John Wilkinson earlier this year, after receiving the challenge from his consultant: https://www.projectnightingale.org/blogs/john-wilkinson-no-holding-us-back/. Thank you so much for sharing your experiences John!
Thank you for reading and have a fabulous rest of the weekend everyone.
Best wishes,
The Project Nightingale Team
Physical activity: high-intensity exercise, inpatient physiotherapy, and optimisation of exercise behaviour
How Do Patients With Axial Spondyloarthritis Experience High-Intensity Exercise? April 2020. Bilberg and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7164628/#acr211128-bib-0020
Although there is growing evidence to show the benefits of high-intensity exercise within Rheumatology, it remains a rather new potential treatment modality. One recent randomised controlled trial (RCT – generally referred to as the “gold standard” for testing treatments) found that high‐intensity exercise improved cardiovascular health and function and reduced disease symptoms (pain, fatigue, stiffness) and inflammation in 100 people with axSpA (see link for full publication: https://pubmed.ncbi.nlm.nih.gov/30745314/). The present paper by Bilberg and colleagues interviewed some of the patients participating in the RCT to gain an understanding of their experience of the programme.
To summarise, supervised high‐intensity interval exercise was perceived as challenging for both body and mind but was also described as a positive experience, with rapid bodily effects that strengthened respondents’ faith in their own bodies. The new experience seemed to have changed the respondents’ attitude and motivation for exercise and made them start taking charge of their health by challenging the disease. Exercise in a social context, under professional leadership, enhanced exercise self‐efficacy and helped the respondents to adhere to the exercise program.
Evaluation of a special concept of physical therapy in spondyloarthritis: German multimodal rheumatologic complex treatment for spondyloarthritis. May 2020. Klemm and colleagues. https://doi.org/10.1007/s10067-019-04887-6
Key Points
- Physical treatment is a key component in treating SpA.
- Multimodal rheumatologic complex treatment (MRCT) is a specific concept of German inpatient care focusing on physical therapy for patients with rheumatic diseases suffering from exacerbated pain and functional impairment. Similar to the 2-week rehabilitation course at the RNHRD, now offered as a virtual course during the pandemic (see our rehabilitation page for more information: https://www.projectnightingale.org/rehabilitation/.)
-For each patient, an individual treatment program is put together based on the patient’s profile of functional impairment, disease activity and comorbidities.
-MRCT has to be provided by a multidisciplinary team lead by a rheumatologist, and treatment modalities have to be chosen from at least three of the following fields: physical therapy, occupational therapy, psychotherapy, pain therapy and behavioural therapy.
-Duration of MRCT can vary depending on medical need, and three options for duration of treatment have been defined by the German medical procedure classification system: 7 to 13 days, 14 to 20 days and more than 21 days of treatment.
-MRCT not only decreases pain and improves function but also reduces disease activity in patients with axSpA, nr-axSpA and axPsA irrespective of the course of disease and comorbidities (e.g. fibromyalgia).
-MRCT could be a role model of treating SpA by means of physical therapy as its effects are not influenced by therapy, disease duration or comorbidities and it has no side effects.
How to optimize exercise behavior in axial spondyloarthritis? Results of an intervention mapping study. May 2020. Hilberdink and colleagues. https://www.sciencedirect.com/science/article/pii/S0738399119305610?via%3Dihub
You may have already seen this article discussed in our December 2019 research blog post – again, with a focus on physical activity. As this article was published as an online pre-print last year. For many of us it can be hard to engage in adequate/recommended exercise, despite its proven health benefits. This study by Hilberdink and colleagues aimed to identify components needed to optimise exercise behaviour in people with axSpA.
The first three steps of the Intervention Mapping protocol used: 1) needs assessment; 2) identification of axSpA-specific exercise barriers and facilitators; 3) selection of effective intervention components addressing potentially modifiable barriers/facilitators. All three steps included reviews of the literature and semi-structured interviews with both people with axSpA and physical therapists.
Results showed that only a reported one third of people with axSpA currently exercise regularly, demonstrating especially a lack of strengthening and cardiorespiratory exercises. Several intervention components were then selected: education, motivational interviewing, goal setting, action planning, monitoring, feedback, tailoring, guided practice, therapists’ training and group exercise encouragement. Research such as this will hopefully provide a foundation for the development of more axSpA-specific exercise interventions, similar to the 2-week rehabilitation course in Bath. There is such a buzz in this area at the moment, especially regarding the development of digitally delivered programs.
Obesity and axSpA outcomes: disease activity, spinal stiffness and comorbidities
Association of body mass index on disease activity in axial spondyloarthritis: systematic review and meta-analysis. May 2020. Liew and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7299511/
In the present study, the authors performed a systematic review of all existing literature evaluating body mass index (BMI – a measure used to see if someone is a healthy weight for their height) and disease activity in axSpA. Before using all the evidence to calculate summary mean (average) differences, comparing obese (BMI>30 kg/m2) or overweight/obese (BMI>25 kg/m2) individuals to those with normal BMI (18.5–24.9 kg/m2).
Twelve studies were included in the meta-analysis. Among all studies reporting disease activity at baseline, the pooled standardised mean difference of disease activity for those with an obese or overweight/obese BMI compared to a normal BMI indicated a significant association of higher BMI with higher disease activity score.
These results demonstrate an association between an overweight/obese BMI and higher disease activity in studies of axSpA. Future longitudinal (long-term) studies of BMI and disease activity should assess how this association changes over time.
Key messages
What is already known about this subject?
Obesity is associated with a poorer response to biological therapy in axial spondyloarthritis (axSpA); higher body mass index (BMI) may be associated with higher disease activity overall.
What does this study add?
This systematic review and meta-analysis demonstrates that higher BMI is significantly associated with higher disease activity in patients with axial spondyloarthritis (axSpA).
How might this impact on clinical practice or future developments?
Interventions on weight loss among those with overweight or obese BMIs should be considered in addition to directed therapies for axSpA, to help reduce disease activity and thereby improve outcomes for patients.
Obesity Increases Disease Activity of Norwegian Patients with Axial Spondyloarthritis: Results from the European Map of Axial Spondyloarthritis Survey. June 2020. Bindesbøll and colleagues.
This study published in June, adds to the evidence presented in the previous study: whereby in 509 participants with axSpA in Norway, obesity was associated with higher reported disease activity score, and being overweight or obese was associated with a higher degree of spinal stiffness and number of comorbidities compared to under/normal weight respondents. These results highlight the serious impact of obesity on health status, and obesity should therefore be considered as a modifiable risk factor for disease activity within the disease management of axSpA.
Smoking: axSpA outcomes and response to TNF inhibitors
Do Smoking and Socioeconomic Factors Influence Imaging Outcomes in Axial Spondyloarthritis? Five-Year Data From the DESIR Cohort. June 2020. Nikiphorou and colleagues. https://onlinelibrary.wiley.com/doi/10.1002/art.41408
In total, 406 axial SpA patients were included (52% male, 40% smokers, and 18% blue collar – i.e. manual labour job). Smoking was independently associated with more magnetic resonance imaging (MRI)‐detected sacroiliac joint (SI joint - joint at the back of the pelvis) inflammation at each visit over the 5 years, an effect that was seen only in patients with blue‐collar professions and in patients with low education levels. Smoking was also significantly associated with spinal inflammation and SI joint damage across all patients, irrespective of socioeconomic factors and other potential confounders.
To summarise, strong associations were found between smoking at baseline and MRI‐detected SI joint inflammation at each visit over a time period of 5 years in axial SpA patients with a blue‐collar job or low education level. These findings suggest a possible role for mechanical stress amplifying the effect of smoking on axial inflammation in axial SpA.
The impact of smoking on response to TNF inhibitors in axial spondyloarthritis: methodological considerations for longitudinal observational studies. April 2020. Zhao and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6694004/
You may also remember the paper discussed in our Part One research updates, published by Zhao and colleagues, looking at smoking and response to TNF inhibitors in axSpA (we thought this deserved and extra mention here, as fitting with the theme of smoking!). Baseline smoking status was associated with significantly worse disease severity at baseline across all measures (disease activity, function, sleep, anxiety, depression). However, response to the first TNF-alpha inhibitor did not differ significantly according to baseline smoking status.
The authors conclude that prescribers should dispel any subconscious bias that smokers may not respond as well to treatment. Nevertheless, emphasise the importance of smoking cessation, particularly given the high burden of heart disease in rheumatic patients. Furthermore, smoking is associated with more severe disease (activity, functional impairment and radiographic progression); although it is not known whether cessation leads to improvement in disease outcome. Conflicting results from prior studies are likely explained by methodological differences.
An update on biomarkers for axSpA
As a quick introduction, a biomarker is a naturally occurring molecule, gene, or characteristic by which a particular pathological or physiological process, disease, etc. can be identified.
HLA-B27 and CRP blood serum biomarkers are currently commonly used in axSpA, whereby there is a strong genetic association of axSpA with the HLA-B27 gene. However, not all axSpA patients are HLA-B27 positive and this often leads to delayed diagnosis in HLA-B27-negative patients. Furthermore, CRP, despite being a widely used laboratory marker for axSpA and used as a criterion for determining treatment, is thought to be lacking in sensitivity and responsiveness, while the natural degree of fluctuation is not well understood. Therefore, no accurate biomarkers or immune-phenotyping tools currently exist for the identification of axSpA.
Identification of appropriate biomarkers in axSpA may in future allow for earlier diagnosis and treatment, better targeting of treatments to those at increased risk of long-term complications and, potentially, better selection of those treatments on the basis of probability of response to specific agents – wouldn’t that be incredible!
Between April and June, we have picked out below two key review articles providing a brief update into research of potential future biomarkers for axSpA.
An update on serum biomarkers to assess axial spondyloarthritis and to guide treatment decision. June 2020. Lorenzin and colleagues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7315656/
This review provided an overview of existing research attempting to identify biomarkers for axSpA. Numerous studies have been conducted to seek biomarkers to guide treatment in SpA and to monitor the disease. However, currently, no new biomarkers satisfy the characteristics for use in clinical practice and CRP remains the most relevant biomarker in axSpA.
Grouping and understanding the role of all other potential biomarkers is challenging. Inflammatory markers have been investigated but standardization is lacking and they mirror CRP. Markers of bone metabolism have shown diverging results on disease activity and progression. The reason could be that biomarkers implicated in the axSpA disease process are restricted to specific tissues and do not migrate to the general circulation. Anti-drug antibodies (produced by the immune system and inactivating the effects of the treatment) and therapeutic drug monitoring are adequate tools to drive treatment decision but are not useful to characterise individuals and identify those prone to more rapid structural damage.
Eventually, a combination of biomarkers may be promising, nevertheless association with clinical characteristics is necessary to increase their predictive value. The authors suggest that further research should focus on the most promising biomarkers in order to reduce the wide variation of observations and better define their role and cut-offs either alone or in combination.
A schematic summary of the potential use of selected promising biomarkers in axial spondyloarthritis.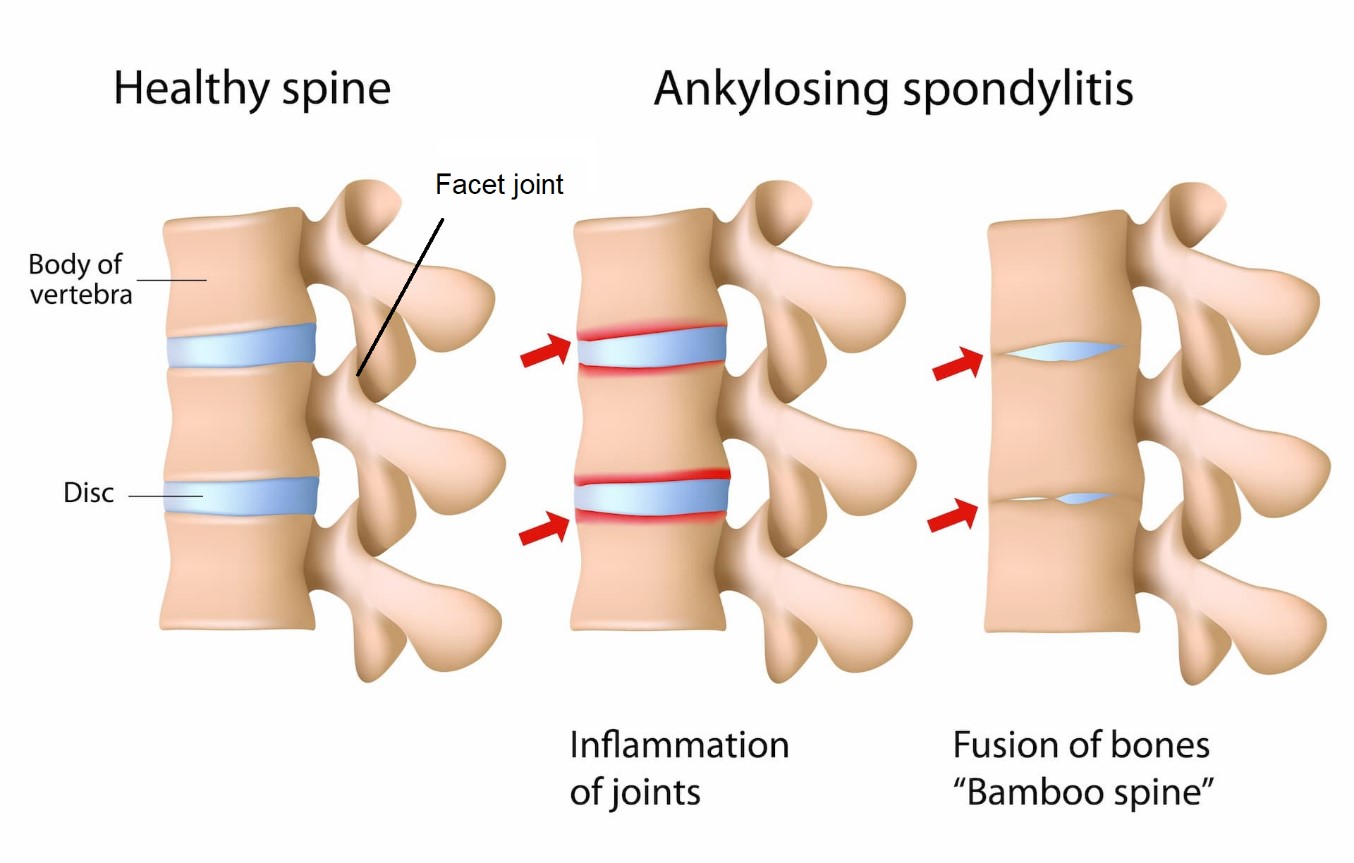
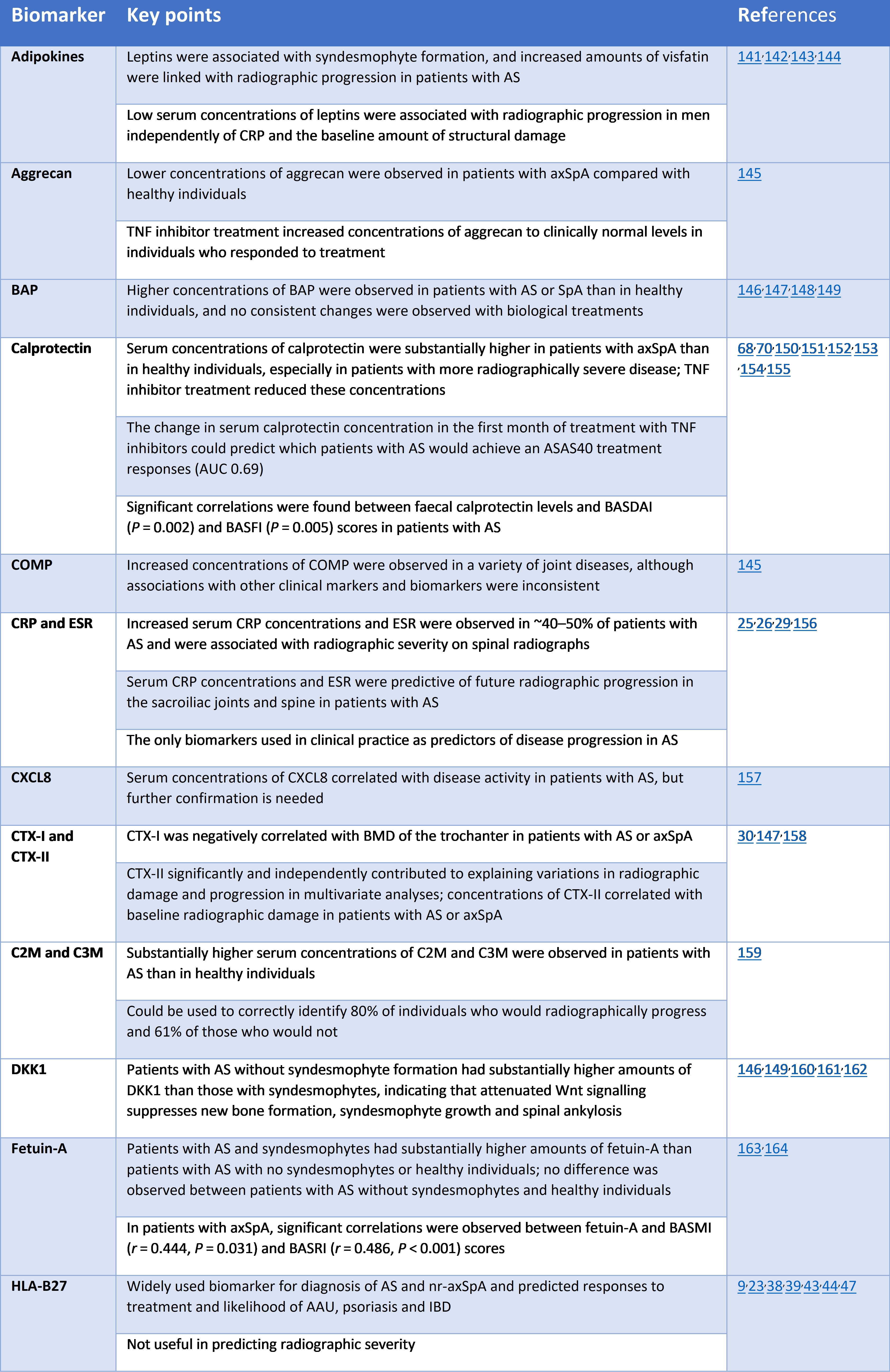
Biomarker development for axial spondyloarthritis. June 2020. Brown and colleagues. https://www.nature.com/articles/s41584-020-0450-0
And a second axSpA biomarker review article in one month! This review by Brown and colleagues was published in the prestigious journal Nature Reviews Rheumatology. As above, the authors state that a combination of biomarkers may have the potential to be more informative than individual biomarkers; however, this is an underexplored area in axSpA and should be investigated further.
Indeed, as outlined above, few biomarkers are in use in clinical practice for axSpA, despite substantial efforts in biomarker discovery to assist with diagnosis, prediction of disease course or therapeutic response. The authors suggest that the reasons for the low rate of translation of this discovery research into clinical practice largely relate to challenges and weaknesses in study design. And therefore conclude that biomarker development programmes should be an integral component of clinical trials, and biomarker data from those trials should be made publicly available for biomarker development research - whereby large publicly-available datasets are required to establish successful biomarker discovery and validation programmes. Multidisciplinary research efforts that bring together teams of researchers with the diverse skills required to make optimal use of the resources available will be critical to ultimately develop a suite of biomarkers for guiding the clinical management of axSpA. Such a development would bring about a major change in clinical practice for individuals with the condition, enabling earlier diagnosis and treatment, better targeting of treatments to those at increased risk of long-term complications and, potentially, better selection of those treatments on the basis of probability of response to specific agents. What a fantastic outcome that would be!
The table below provides an overview of some of the investigated biomarkers for diagnosis, progression and response to treatment in axSpA. Interestingly, in the text, the authors also extensively discuss the potential of the gut microbiome (huge numbers of microorganisms and their genetic material, involved in functions critical to your health and wellbeing) for future diagnostic use. Whereby it could potentially also have predictive value for clinical manifestations and disease course. Again, the authors highlight the potential for use of combined biomarkers: suggesting that genetic profiling and microbiome profiling could have added value when used together for axSpA diagnosis.
Key investigated biomarkers for diagnosis, disease progression and response to treatment in axSpA.